There are a little over 90 naturally occurring elements found on our planet and the vast majority of these are native metals – metals that are found on earth in their metallic form. However, despite the large number of different available metals on our earth, in their purest form they are less than ideal for the tasks that we need them for in today’s modern age.
In order to produce metals that are suitable for our uses, we take naturally occurring metals and combine them in specific quantities to produce Alloys. An alloy is simply a mixture of metals (and sometimes non-metallic elements) to produce another material more suitable to our needs. This is generally done to improve its ‘engineering properties’, such as tensile strength, ductility, shear strength, hardness, etc. The product of the smelting together of two elements is often improved over the individual elements.
One of the first alloys to be created was Bronze and sparked what we now call the Bronze Age (as early as 3300BC). Bronze is a combination of Copper and Tin, two naturally occurring metals common in the ground and at the time was the hardest metal available. Copper and tin are smelted together, most commonly using 88% copper and 12% tin. The smelting together of these two metals produced Bronze, a metal that was harder and easier to cast than either of the two separate ingredients.

In today’s age, the most common alloys available are alloys of iron and, more recently, aluminium. Iron has some of the most commonly understood alloys, such as Steel. Steel is an alloy of iron, created by smelting Iron with carbon at around 2.1%. The introduction of carbon into the alloy massively improves the hardness of the product, resulting in what we know today as steel – a material known for its versatility, strength and heat treating properties. Later, other alloys of iron and steel became available such as High Carbon Steel and Stainless Steel.
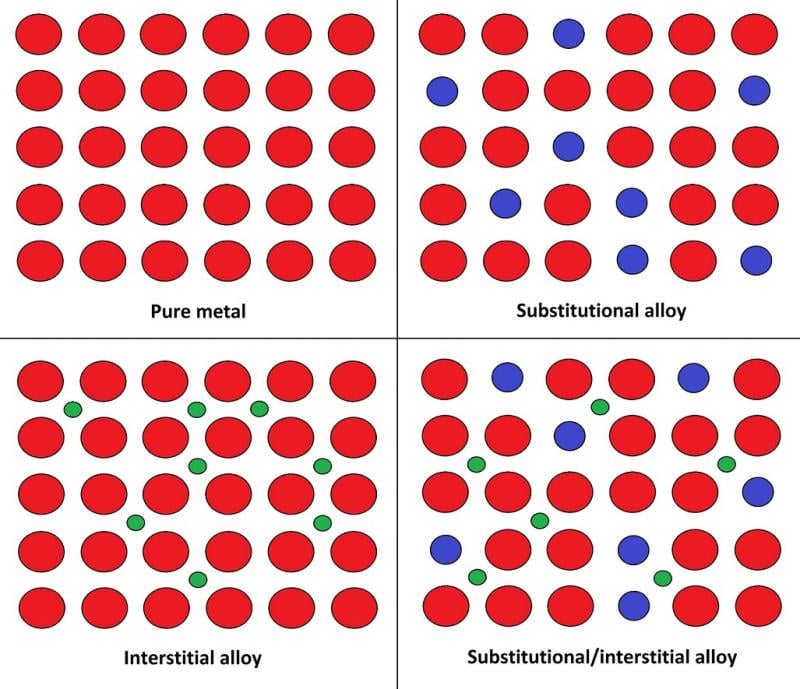
Alloys generally hold better engineering qualities when compared to ‘pure’ metals due to their atomic structure. Pure metal consists of identical atoms arranged in regular layers, this allows each layer to slide and disconnect from the next easily. When we introduce another element, it sits between any gaps in the metal atom’s layers and makes the metallic structure much harder to separate.
At MRT, we specialise predominantly in non-ferrous alloys, such as aluminium and zinc, each with a number of different alloys available for different potential uses.
Each aluminium alloy we offer is suited to different uses, be it machinability like alloy LM5, casting techniques like LM6 and LM25, corrosion resistance like LM31 or a combination of different characteristics. For a full list of the aluminium alloys available at MRT click below.
Zinc alloys are also available for casting at MRT. These alloys are twice as dense as aluminium, with excellent non-spark characteristics. Zinc alloys such as ZL27 can be considered for applications whose service temperatures are as high as 150°C and have excellent machining characteristics due to the 25% aluminium composition.










